We use cookies to make your experience better. To comply with the new e-Privacy directive, we need to ask for your consent to set the cookies. Learn more.
Background
Papain (papaya proteinase I) is a non-specific thiol-endopeptidase, active only when the sulfhydryl group of the Cys25 residue within its active site is in its reduced form.
During the proteolytic activity this cysteine residue is first deprotonated by the imidazole group of a surrounding histidine residue, and then attacks the peptide bond within its specific amino acid pattern to form an acyl-enzyme intermediate. The covalent link is then hydrolysed and both enzyme and truncated peptide are released.
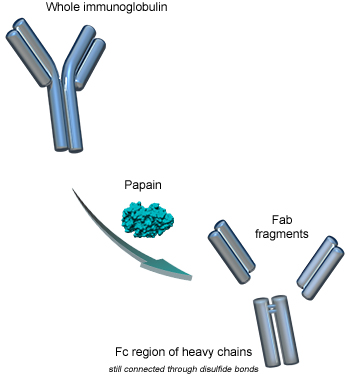
Digestion of immunoglobulins
When immunoglobulins are incubated with papain in the presence of a reducing agent, several peptide bonds above the hinge region are hydrolysed, producing three fragments of similar size (50kDa):
- two monovalent Fab fragments
- one Fc fragment
Use of Fab fragments
Compared to whole IgG, Fab fragments can penetrate cells and tissues and bind antigens more easily thanks to their lower steric hindrance. Moreover, the absence of Fc region prevents Fab fragments from nonspecific binding to Fc receptors.
When the Fc fragment is of interest, papain is consequently the enzyme of choice. Papain is primarily used to generate Fab fragments, but it can also be used to generate F(ab’)2 fragments when used in specific physico-chemical conditions.