We use cookies to make your experience better. To comply with the new e-Privacy directive, we need to ask for your consent to set the cookies. Learn more.

Coupling Principle
In the case of antibodies, the position of the label must be carefully considered so as not to interfere with the antigen binding sites. Through our expertise in various coupling chemistries, we are able to choose the position of the label in order to maintain both antibody immunoreactivity and label activity. For each project immunoreactivity of the conjugated antibody as well as the activity of the conjugate are strictly controlled (i.e. enzymatic activity when coupling to an enzyme).
Enzymes can be covalently coupled to a specific antibody using maleimide-sulfhydryl coupling chemistries. Succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (Sulfo-SMCC) popular crosslinking reagent can be used to produce antibody-enzyme conjugates as well as hapten-carrier conjugates (see example below).
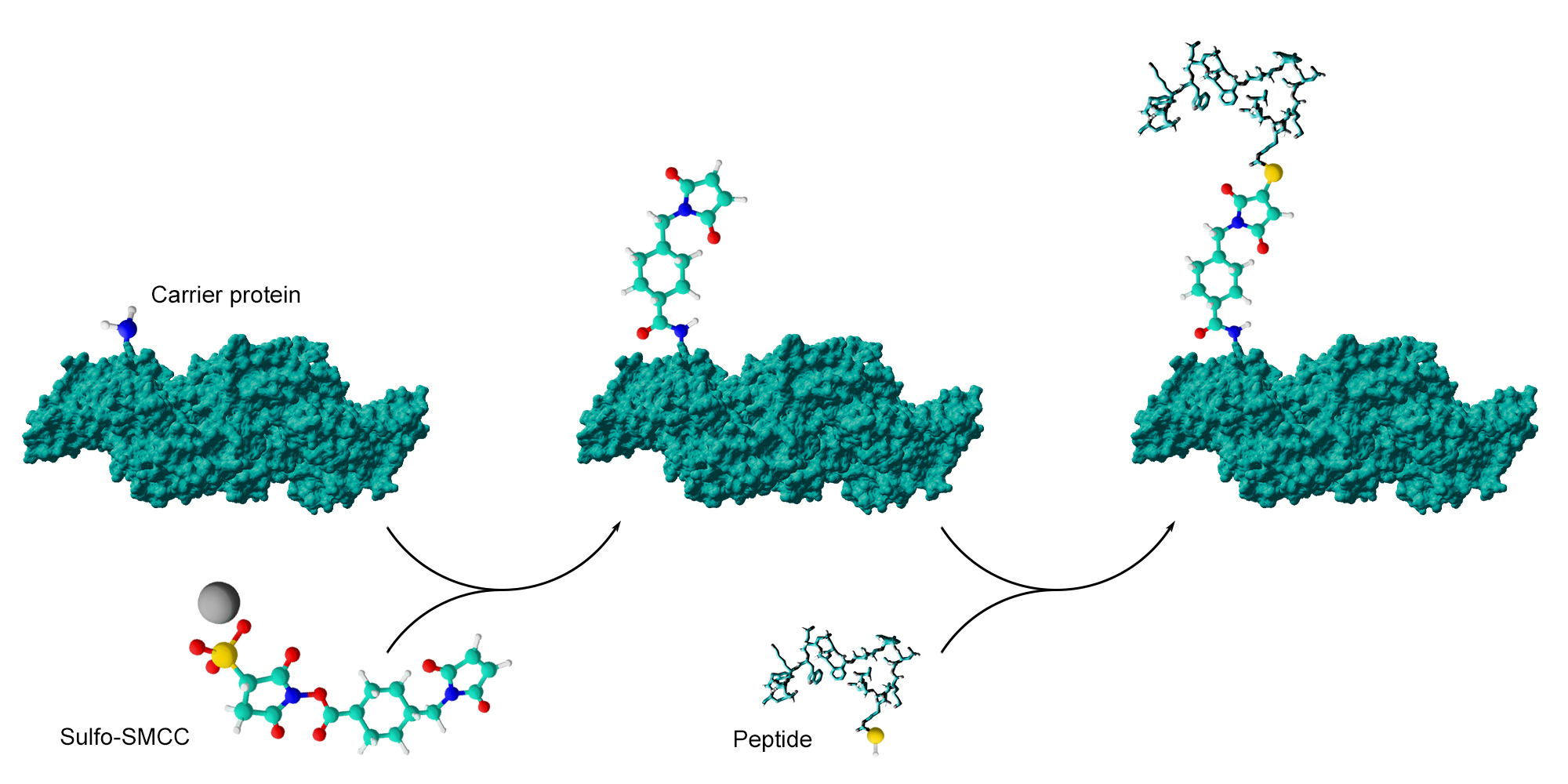
Reaction between sulfo-SMCC crosslinker and a free amine of the carrier protein or antibody forms a maleimide-activated sulfhydril-reactive molecule. The maleimide functional group then forms a crosslink with reduced sulfhydril groups within immunogenic peptides (cysteine residues) or haptens (free thiols). Carrier proteins can be activated by several maleimide groups and then carry several conjugated peptides or haptens.
Periodate oxidation methods, which specifically direct the coupling location to the Fc portion of the antibody molecule, may also be used. Attachment through this location minimises the steric hindrance with regard to the antigen bindind sites of the antibody.
